Does Calcium Bind To Troponin Or Tropomyosin
pinupcasinoyukle
Nov 13, 2025 · 12 min read
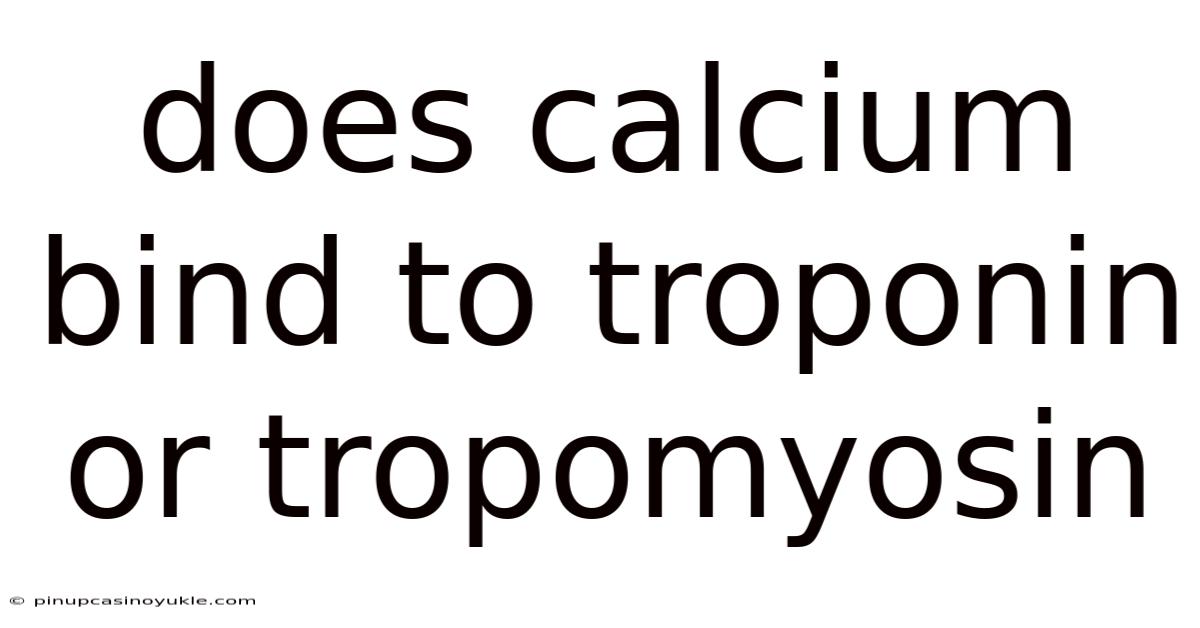
Table of Contents
Calcium's pivotal role in muscle contraction often leads to the question: does calcium bind to troponin or tropomyosin? The accurate answer is troponin. This interaction initiates a cascade of events that ultimately allow for muscle contraction. To fully understand this process, it's essential to delve into the intricacies of muscle structure, the roles of troponin and tropomyosin, and the specific mechanism by which calcium triggers contraction.
The Basics of Muscle Structure
Muscles, the engines of movement, are composed of bundles of muscle fibers. Each muscle fiber contains myofibrils, which are the contractile units. Myofibrils are made up of repeating segments called sarcomeres, the functional units of muscle contraction. Within the sarcomere, two primary protein filaments are found:
- Actin: Thin filaments that contain binding sites for myosin.
- Myosin: Thick filaments with "heads" that bind to actin, enabling muscle contraction.
These filaments interact to generate force, but this interaction is carefully regulated by two other proteins: troponin and tropomyosin.
Troponin and Tropomyosin: The Gatekeepers of Muscle Contraction
-
Tropomyosin is a long, fibrous molecule that wraps around the actin filament. In a resting muscle, tropomyosin physically blocks the myosin-binding sites on actin, preventing the formation of cross-bridges and thus preventing contraction.
-
Troponin is a complex of three proteins (Troponin T, Troponin I, and Troponin C) that is attached to tropomyosin.
- Troponin T (TnT) binds the troponin complex to tropomyosin.
- Troponin I (TnI) inhibits the binding of myosin to actin.
- Troponin C (TnC) binds calcium ions and plays a central role in initiating muscle contraction.
The Role of Calcium: Unlocking Muscle Contraction
When a nerve impulse reaches a muscle fiber, it triggers the release of calcium ions (Ca2+) from the sarcoplasmic reticulum, a specialized endoplasmic reticulum in muscle cells. This release dramatically increases the calcium concentration in the vicinity of the myofibrils. Here's where the crucial interaction takes place:
- Calcium Binding: Calcium ions bind to troponin C (TnC). This binding is specific and essential for initiating muscle contraction.
- Conformational Change: The binding of calcium to TnC induces a conformational change in the troponin complex. This change is like a molecular switch being flipped.
- Tropomyosin Shift: As troponin changes shape, it pulls tropomyosin away from the myosin-binding sites on actin. This uncovers the binding sites, making them accessible to myosin heads.
- Cross-Bridge Formation: With the binding sites exposed, myosin heads can now attach to actin, forming cross-bridges.
- Power Stroke: Once the cross-bridge is formed, myosin heads pull the actin filaments towards the center of the sarcomere, shortening the sarcomere and generating force. This is known as the power stroke.
- Muscle Contraction: The collective shortening of sarcomeres throughout the muscle fiber results in muscle contraction.
In essence, calcium acts as the key that unlocks the muscle contraction machinery. By binding to troponin, it triggers a series of events that ultimately allow myosin to bind to actin and initiate the sliding filament mechanism.
Why Troponin and Not Tropomyosin?
While tropomyosin is directly involved in blocking the myosin-binding sites, it doesn't directly bind calcium. The regulation of tropomyosin's position is controlled by the troponin complex. Troponin acts as the calcium sensor, and its conformational change dictates whether tropomyosin exposes or blocks the binding sites.
Think of it like a lock and key: calcium is the key, troponin is the lock mechanism that responds to the key, and tropomyosin is the door that either opens or closes the pathway for muscle contraction.
The Molecular Details of Troponin-Calcium Interaction
Troponin C (TnC) is a dumbbell-shaped protein with two globular domains, each containing two calcium-binding sites. These sites are categorized as either high-affinity or low-affinity sites.
- High-Affinity Sites: These sites are always occupied by calcium (or magnesium) even at resting calcium concentrations. They play a structural role in maintaining the integrity of the troponin complex.
- Low-Affinity Sites: These sites only bind calcium when calcium levels rise during muscle stimulation. It is the binding of calcium to these low-affinity sites that triggers the conformational change in troponin, leading to muscle contraction.
The binding of calcium to TnC causes a rearrangement of the interactions between the troponin subunits. Specifically, it weakens the interaction between TnI (the inhibitory subunit) and actin, allowing TnT to pull tropomyosin away from the myosin-binding sites.
Relaxation: Reversing the Process
Muscle relaxation occurs when nerve stimulation ceases, and calcium is actively pumped back into the sarcoplasmic reticulum by a calcium ATPase pump. As calcium levels in the cytoplasm decrease, calcium detaches from troponin C. This causes troponin to return to its original conformation, allowing tropomyosin to slide back into its blocking position over the myosin-binding sites on actin. With the binding sites blocked, myosin can no longer bind to actin, cross-bridges detach, and the muscle relaxes.
Clinical Significance
The troponin complex, particularly troponin I and troponin T, has significant clinical relevance in the diagnosis of myocardial infarction (heart attack). When heart muscle is damaged, troponin is released into the bloodstream. Elevated levels of troponin in the blood are a sensitive and specific indicator of heart muscle damage. This is because cardiac troponin isoforms are slightly different from skeletal muscle troponin, allowing for specific detection in blood tests.
Beyond Skeletal Muscle: Smooth Muscle Contraction
While the troponin-tropomyosin system is central to skeletal and cardiac muscle contraction, smooth muscle employs a different mechanism for regulating contraction. Smooth muscle lacks troponin. Instead, calcium binds to calmodulin, a calcium-binding protein. The calcium-calmodulin complex then activates myosin light chain kinase (MLCK), which phosphorylates myosin light chains. This phosphorylation enables myosin to bind to actin and initiate contraction.
The Importance of Understanding the Calcium-Troponin Interaction
Understanding the precise mechanism by which calcium interacts with troponin is crucial for several reasons:
- Drug Development: It provides targets for developing drugs that can modulate muscle contraction. For example, drugs that enhance calcium sensitivity of troponin could be used to improve cardiac function in heart failure patients.
- Understanding Muscle Disorders: It helps in understanding the pathophysiology of various muscle disorders, such as hypertrophic cardiomyopathy, where mutations in troponin genes can lead to abnormal muscle contraction.
- Improving Athletic Performance: A deeper understanding of muscle physiology can aid in developing training strategies and nutritional interventions to optimize muscle function and athletic performance.
In Summary: The Calcium-Troponin-Tropomyosin Dance
To recap, calcium does indeed bind to troponin, specifically to troponin C (TnC). This interaction is the linchpin of muscle contraction in skeletal and cardiac muscle. The binding of calcium to TnC triggers a conformational change in the troponin complex, which in turn moves tropomyosin away from the myosin-binding sites on actin. This allows myosin to bind to actin, forming cross-bridges and initiating the sliding filament mechanism that drives muscle contraction. When calcium levels decrease, the process reverses, leading to muscle relaxation. This intricate dance between calcium, troponin, and tropomyosin is essential for all voluntary and involuntary movements, from walking and breathing to the beating of our hearts.
Elaborating on the Troponin Subunits
To appreciate the complexity of the troponin complex, let's delve deeper into the individual roles of its three subunits: Troponin T (TnT), Troponin I (TnI), and Troponin C (TnC).
Troponin T (TnT): The Tropomyosin Anchor
TnT is the largest subunit of the troponin complex and plays a crucial role in anchoring the entire complex to tropomyosin. Its primary function is to bind the troponin complex to tropomyosin, forming a stable regulatory unit along the actin filament. TnT has a long, elongated structure that allows it to interact with multiple regions of tropomyosin. Different isoforms of TnT exist, and their expression varies depending on the muscle type (skeletal, cardiac, or smooth). These isoforms can modulate the calcium sensitivity of the muscle. Mutations in TnT have been linked to various muscle disorders, including hypertrophic cardiomyopathy and dilated cardiomyopathy.
Troponin I (TnI): The Inhibitor of Contraction
TnI is the inhibitory subunit of the troponin complex. Its main function is to prevent muscle contraction in the absence of calcium. TnI achieves this by binding to actin and blocking the interaction between actin and myosin. Specifically, TnI interacts with actin in a way that sterically hinders the binding of myosin heads to their binding sites on actin. When calcium binds to TnC, the interaction between TnI and actin is weakened, allowing tropomyosin to move away and enabling contraction. Cardiac TnI (cTnI) is a specific isoform found only in heart muscle, making it a valuable marker for detecting heart damage.
Troponin C (TnC): The Calcium Sensor
TnC is the calcium-binding subunit of the troponin complex and the focus of our discussion. It is a dumbbell-shaped protein with two globular domains, each containing two calcium-binding sites. As mentioned earlier, these sites are classified as high-affinity and low-affinity sites. The high-affinity sites are always occupied and play a structural role, while the low-affinity sites bind calcium only when calcium levels rise, triggering the conformational change that initiates muscle contraction. TnC is structurally similar to calmodulin, another calcium-binding protein involved in various cellular processes. In skeletal muscle, TnC has two isoforms: slow skeletal TnC and fast skeletal TnC.
Expanding on the Sliding Filament Theory
The interaction between actin and myosin, regulated by troponin and tropomyosin, is the basis of the sliding filament theory of muscle contraction. This theory, proposed by Andrew Huxley and Ralph Niedergerke, explains how muscles generate force and shorten.
- Resting State: In a resting muscle, tropomyosin blocks the myosin-binding sites on actin. Myosin heads are energized and ready to bind, but they are prevented from doing so.
- Activation: When a nerve impulse arrives, calcium is released from the sarcoplasmic reticulum. Calcium binds to TnC, causing a conformational change in the troponin complex.
- Exposure of Binding Sites: The conformational change in troponin moves tropomyosin away from the myosin-binding sites on actin, exposing the sites for myosin to bind.
- Cross-Bridge Formation: Myosin heads bind to the exposed binding sites on actin, forming cross-bridges.
- Power Stroke: Once the cross-bridge is formed, the myosin head pivots, pulling the actin filament towards the center of the sarcomere. This movement is powered by the hydrolysis of ATP (adenosine triphosphate), which provides the energy for the myosin head to change its conformation.
- Detachment: After the power stroke, ATP binds to the myosin head, causing it to detach from actin.
- Re-Energizing: The ATP is hydrolyzed, re-energizing the myosin head and preparing it to bind to another site on the actin filament.
- Cycle Repetition: The cycle of cross-bridge formation, power stroke, detachment, and re-energizing repeats as long as calcium is present and ATP is available. This continuous cycle causes the actin and myosin filaments to slide past each other, shortening the sarcomere and generating force.
Practical Applications and Research Directions
The detailed understanding of the calcium-troponin interaction has led to numerous practical applications and continues to be an active area of research.
- Cardiac Biomarkers: As mentioned earlier, cardiac troponin (cTnI and cTnT) are widely used as biomarkers for diagnosing myocardial infarction. High-sensitivity troponin assays have further improved the accuracy and speed of diagnosing heart attacks.
- Drug Development: Researchers are exploring drugs that can modulate the calcium sensitivity of troponin to improve cardiac function in heart failure patients. These drugs, known as calcium sensitizers, enhance the force of contraction without increasing the calcium concentration in the cell.
- Genetic Mutations: Mutations in troponin genes have been linked to various muscle disorders, including hypertrophic cardiomyopathy (HCM) and dilated cardiomyopathy (DCM). Understanding the effects of these mutations on the structure and function of troponin is crucial for developing targeted therapies.
- Skeletal Muscle Fatigue: The calcium-troponin interaction is also implicated in skeletal muscle fatigue. During prolonged exercise, the calcium sensitivity of troponin can decrease, leading to reduced force production and muscle fatigue.
- Muscle Regeneration: Researchers are investigating the role of troponin in muscle regeneration and repair after injury. Understanding how troponin expression and function are regulated during muscle regeneration could lead to new strategies for treating muscle injuries and diseases.
- Evolutionary Studies: Comparative studies of troponin isoforms in different species can provide insights into the evolution of muscle contraction mechanisms.
Concluding Thoughts
The calcium-troponin-tropomyosin system is a marvel of molecular engineering that enables the precise and coordinated contraction of muscles. Calcium's specific binding to troponin C initiates a cascade of events that ultimately allow myosin to bind to actin and generate force. This intricate dance between calcium, troponin, and tropomyosin is essential for all forms of movement, from the simplest reflexes to the most complex athletic feats. Continued research into the calcium-troponin interaction promises to yield new insights into muscle physiology and new therapies for muscle disorders. Understanding this fundamental process is key to unlocking the secrets of muscle function and improving human health.
Frequently Asked Questions (FAQ)
Q: Does calcium bind directly to tropomyosin?
A: No, calcium does not bind directly to tropomyosin. Calcium binds to troponin C (TnC), which is a component of the troponin complex. The troponin complex then interacts with tropomyosin to regulate its position on the actin filament.
Q: What happens if calcium levels are too low?
A: If calcium levels are too low, calcium will detach from troponin C (TnC). This causes troponin to return to its original conformation, allowing tropomyosin to slide back into its blocking position over the myosin-binding sites on actin. As a result, myosin cannot bind to actin, cross-bridges detach, and the muscle relaxes.
Q: Why is cardiac troponin used as a marker for heart attacks?
A: Cardiac troponin (cTnI and cTnT) are specific isoforms found only in heart muscle. When heart muscle is damaged, troponin is released into the bloodstream. Elevated levels of cardiac troponin in the blood are a sensitive and specific indicator of heart muscle damage, such as that caused by a heart attack.
Q: What is the role of ATP in muscle contraction?
A: ATP (adenosine triphosphate) plays several crucial roles in muscle contraction:
- It provides the energy for the myosin head to pivot and pull the actin filament during the power stroke.
- It binds to the myosin head, causing it to detach from actin after the power stroke.
- It is used by the calcium ATPase pump to actively transport calcium back into the sarcoplasmic reticulum, which is essential for muscle relaxation.
Q: How does smooth muscle contraction differ from skeletal muscle contraction?
A: Smooth muscle contraction differs from skeletal muscle contraction in several key ways:
- Smooth muscle lacks troponin. Instead, calcium binds to calmodulin, a calcium-binding protein.
- The calcium-calmodulin complex activates myosin light chain kinase (MLCK), which phosphorylates myosin light chains. This phosphorylation enables myosin to bind to actin and initiate contraction.
- Smooth muscle contraction is typically slower and more sustained than skeletal muscle contraction.
Latest Posts
Latest Posts
-
How To Find Volume Of A Rectangular Pyramid
Nov 13, 2025
-
What Is The Difference Between An Ectotherm And An Endotherm
Nov 13, 2025
-
What Are Dependent Clauses And Independent Clauses
Nov 13, 2025
-
The Average Rate Of Change Is
Nov 13, 2025
-
How Do We Describe Transformation In Bacteria
Nov 13, 2025
Related Post
Thank you for visiting our website which covers about Does Calcium Bind To Troponin Or Tropomyosin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.